Pipeline
ATA3431
ATA3431: Off-the-Shelf Allogeneic CD19/CD20 Program for B-Cell Hematological and Autoimmune Malignancies
-
-
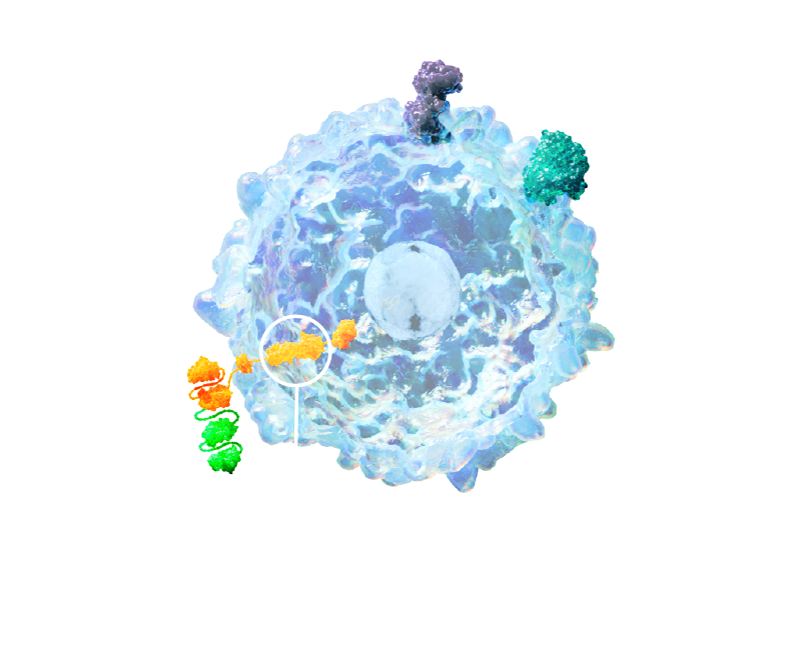
- ATA3431 consists of allogeneic Epstein-Barr virus (EBV)-sensitized T cells that express a CD20/CD19 CAR construct
- The CD20-CD19 binding domains are oriented to optimize potency
- Dual targeting designed to address antigen escape and tumor variability
- ATA3431 manufacturing is based on clonally expanded EBV T cells, inherently having lower potential for alloreactivity
- 1XX signaling domain optimizes expansion and mitigates T-cell exhaustion
- ATA3431 CAR T cells have been designed for T-cell memory, expansion, and anti-tumor efficacy1
- Preclinical data have demonstrated early evidence of antitumor activity, long-term persistence, and superior tumor growth inhibition compared to an autologous CD19/CD20 CAR T benchmark1
- Academic program generated proof-of-principle for an earlier-generation allogeneic CD19 targeted CAR EBV T-cell construct in relapsed/refractory B-cell malignancies after stem cell transplant2
- IND targeted for 2025
- Cha, S et al. Oral Presentation. ATA3431: Allogeneic CD19/CD20 Bispecific CAR EBV T Cells for the Treatment of B-Cell Malignancies. TCT 2024.
- Curran KJ, et al. ASH 2023
ATA3431
Off-the-shelf allogeneic CD19/CD20 CAR T program progressing toward an IND submission in Q4 2025.
- Program/Indication
- Pre-clinical
- Phase 1
- Phase 2
- Phase 3
-
-
- ATA3431 is an allogeneic dual CAR T candidate targeting both CD19 and CD20 antigens for B-cell malignancies.
- Dual targets reduce probability of relapse due to CD19 antigen loss, hypothesized to be a major cause of treatment resistance or disease relapse after CD19 CAR T treatment in B-cell malignancies.
- Features a novel 1XX costimulatory domain, memory phenotype, and retained, unedited T-cell receptor.
-
-
- ATA3431 is an allogeneic dual CAR T candidate targeting both CD19 and CD20 antigens for autoimmune disease.
- Features a novel 1XX costimulatory domain, memory phenotype, and retained, unedited T-cell receptor.
Next-Generation CAR T Technology
Our preclinical pipeline is rapidly expanding with novel technologies and next-generation, multi-targeted CAR T immunotherapies thanks to our collaboration with Memorial Sloan Kettering Cancer Center.