Technology
Cutting-edge allogenic T-Cell medicines, made for rapid availability.
Leveraging the body's honed immune response to EBV.
Diverse applications for allogeneic approach across cancer and autoimmune disease.
Clinically tested platform with inherently low alloreactivity, eliminating need for gene editing that may reduce T-cell fitness.
Leveraging expertise in EBV T-cell biology and next-generation CARs to overcome limitations in current CAR T therapies.
Our Approach
Manufacturing allogeneic EBV T cells
Manufactured from healthy donors to form the basis of a diverse portfolio of investigational therapies.
Collection
Immune cells are collected from healthy donors to help ensure high-quality starting material.
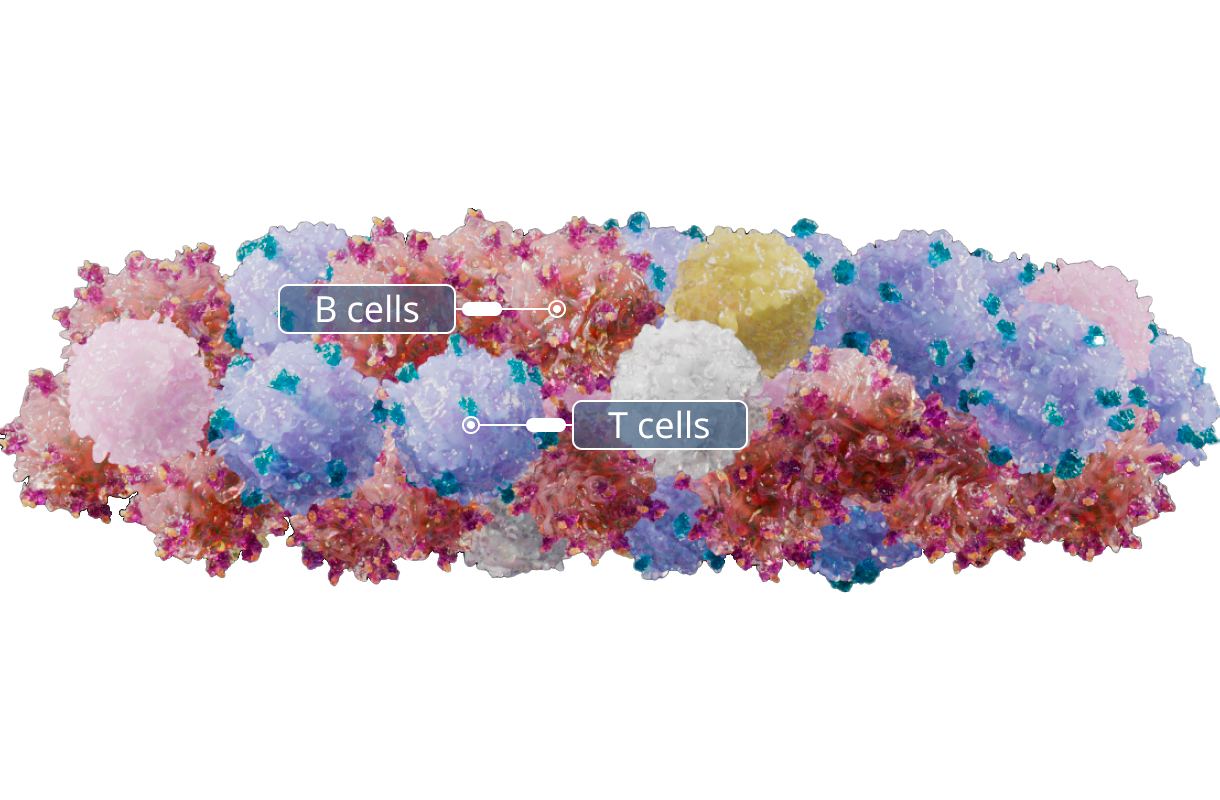
Stimulation
T cells are exposed to EBV antigen presenting B lymphocytes to help enrich for T cells that recognize EBV.
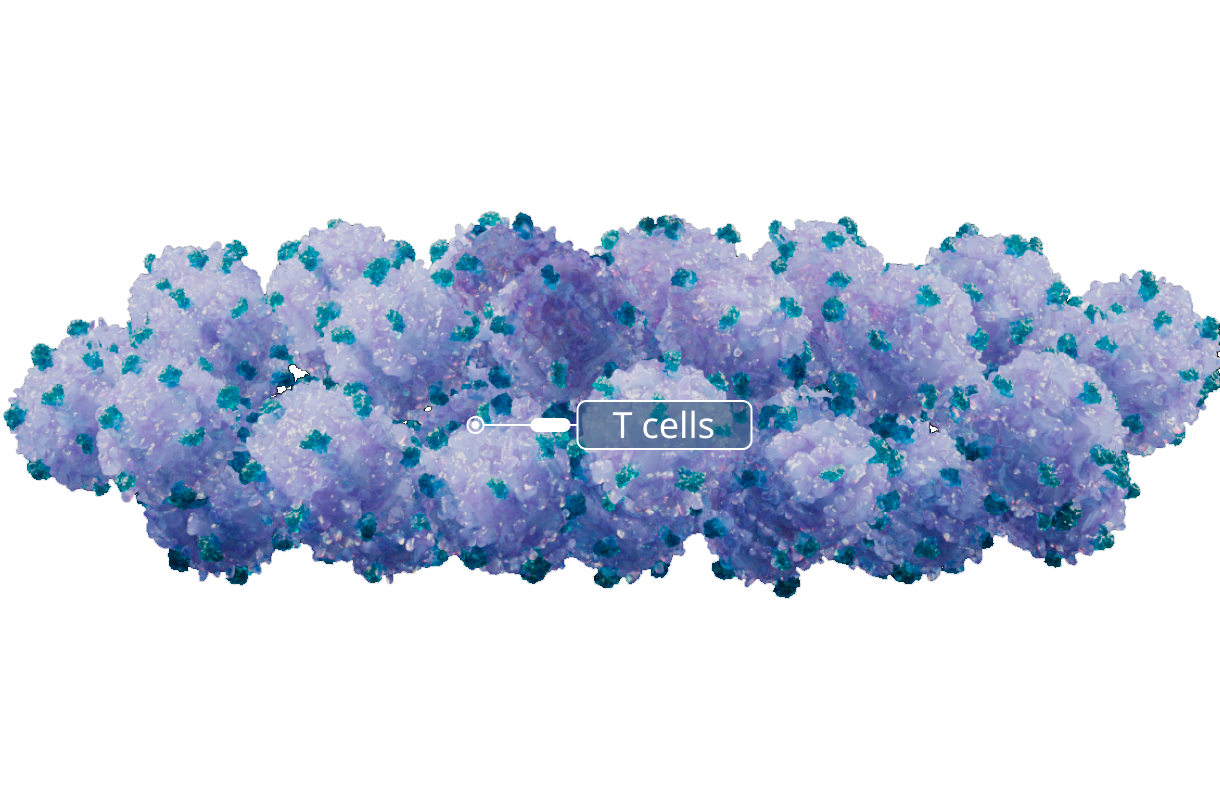
Expansion
T cells that recognize EBV through their T-cell receptor (EBV T cells) are expanded over several rounds of stimulation with EBV antigen presenting B lymphocytes. This approach allows many EBV T cells to be produced from a single donor.
Storage
Once expanded, cells are comprehensively characterized and lots meeting specific release criteria are stored for future use. Our platform allows us to produce our therapies in advance of patient need which can be delivered for use within days.

The resulting cells are enriched to detect and destroy EBV-infected cells or subsequently modified for other applications.
Innovating toward next-generation CAR T cells
Building on our EBV T-cell platform enables us to target diseases not associated with EBV by expressing modified CARs on the surface of the cells.
CAR gene is delivered.
The CAR gene is delivered to an EBV-sensitized T cell via a retroviral vector.
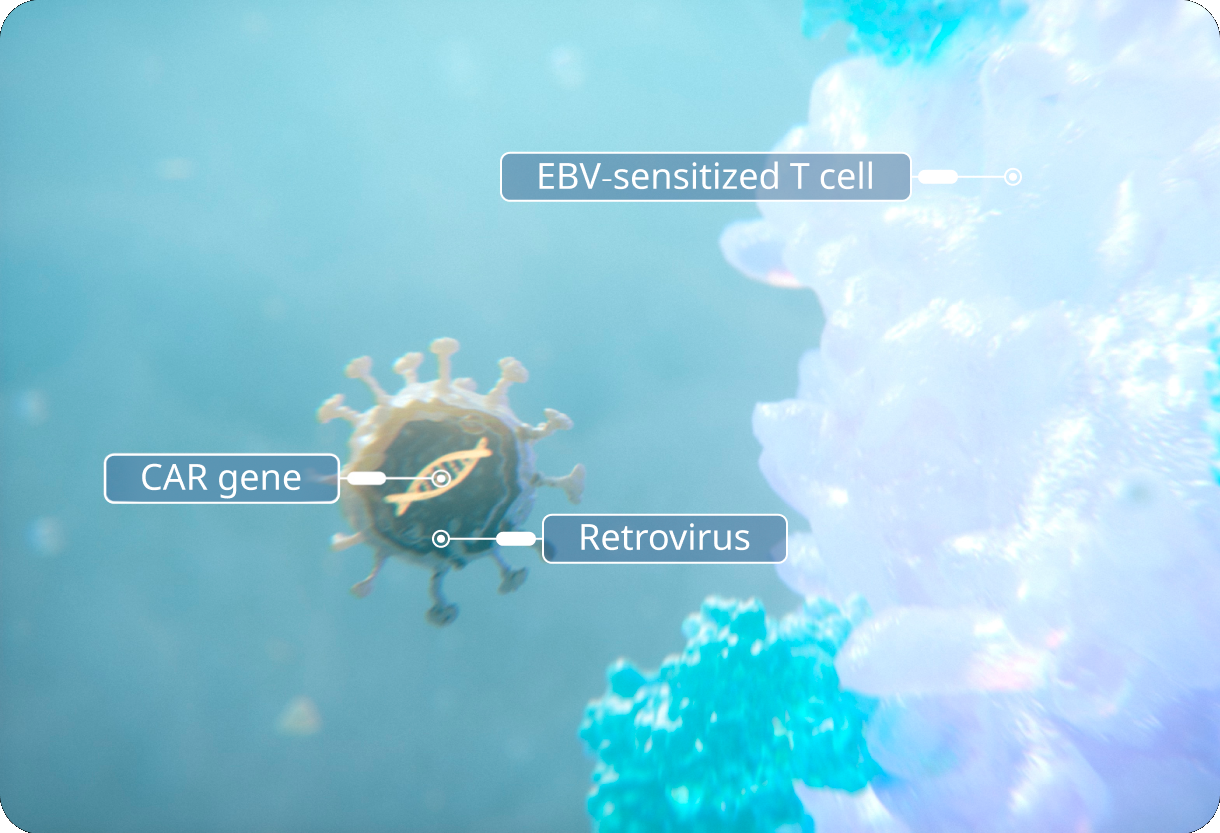
CAR gene inserts into T-cell DNA.
The CAR gene inserts into the DNA of the EBV T cell, where, once expressed, it will generate a CAR domain on its surface that recognizes specific antigens on target cells.
Technologies aiming to enhance CAR T function.
The 1XX costimulatory domain and PD-1 DNR may help reduce T-cell exhaustion, improve persistence, and resist the immunosuppressive tumor environment.
Cells are stored for delivery.
Once the cells have been comprehensively characterized, they are stored in large quantities and ready for rapid delivery to patients as an off-the-shelf treatment.
